
Two-photon imaging of microglial response to photodamage in the brain of a living mouse (Neurotar’s publication).
We use two-photon microscopy to measure microglia dynamics in the most physiologically relevant model system: the brains of awake, behaving mice. We can measure cell morphology, process motility and microglial migration over time frames from minutes to months. Our assay is uniquely suited to longitudinally assess drug efficacy or mode of action, or to characterize mouse disease models. Neurotar’s scientists can combine this assay with other imaging readouts or with simple behavioral readouts.
Microglia are multifunctional immune cells with a clear link to neurological disease
Microglia are the resident immune cells of the central nervous system, and they have multiple functions. They remove pathogens, dead cells, protein aggregates, and other dangers to the CNS, and they are key mediators of neuroinflammation. Interestingly, microglia also carry out several essential non-immune tasks in the brain. For example, microglia regulate dendritic spine plasticity, synaptic remodeling, neurogenesis, maintenance of the blood-brain barrier, and blood vessel structure. Importantly, microglia play an important role in several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, ALS, and Huntington’s disease.
Microglia are highly dynamic cells that form long processes, which constantly extend and retract to survey their surroundings. Upon detection of certain danger signals, microglia change shape and move their cell body toward the affected site, where they destroy the pathogen or remove the debris. In addition, upon certain queues, the dynamic processes can contact neurons and mediate synaptic plasticity. Microglia can mediate synaptic pruning but also trigger the formation of new synapses. Thus, being highly dynamic is essential for microglial function.
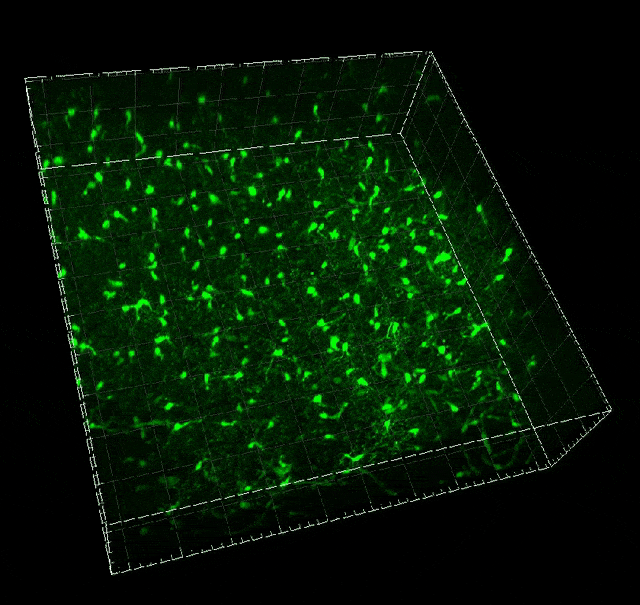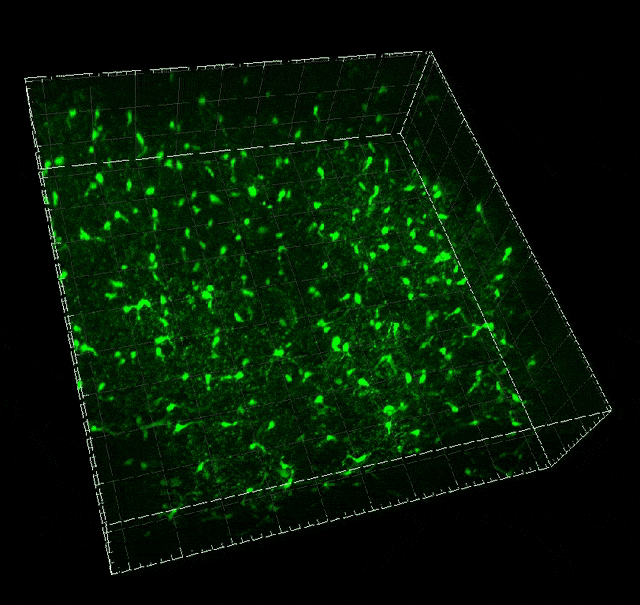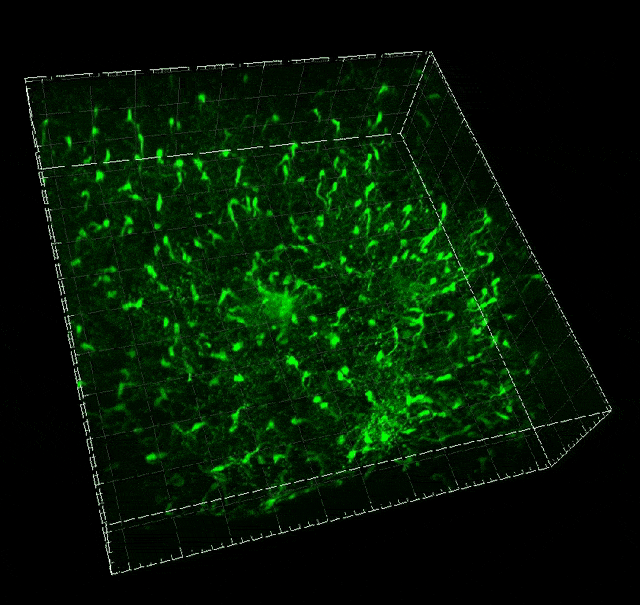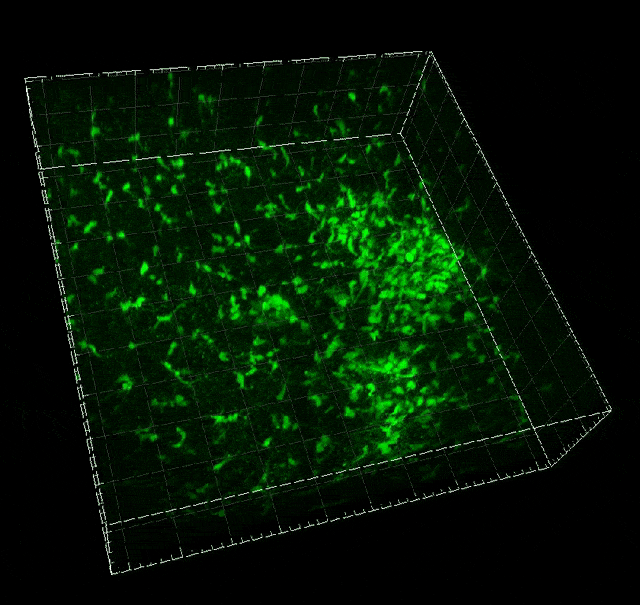
Microglial migration induced by a large laser-induced injury. Time points -30, 20 and 100 min, 24, 48 and 72h. Image obtained at Neurotar.
Why study microglial dynamics using two-photon microscopy?
The brain is composed of a mix of different cell types that constantly signal to each other. Microglial activity and dynamics depend on the integrated signals perceived from those different cells. These signals vary in space and time and are strongly affected by external factors such as stress, pathogens, and age. Although in vitro or ex vivo assays exist to measure microglial dynamics, these simple assays do not capture the complexity and dynamic nature of the brain. Therefore, the living brain is the most physiologically relevant environment to study microglial function.
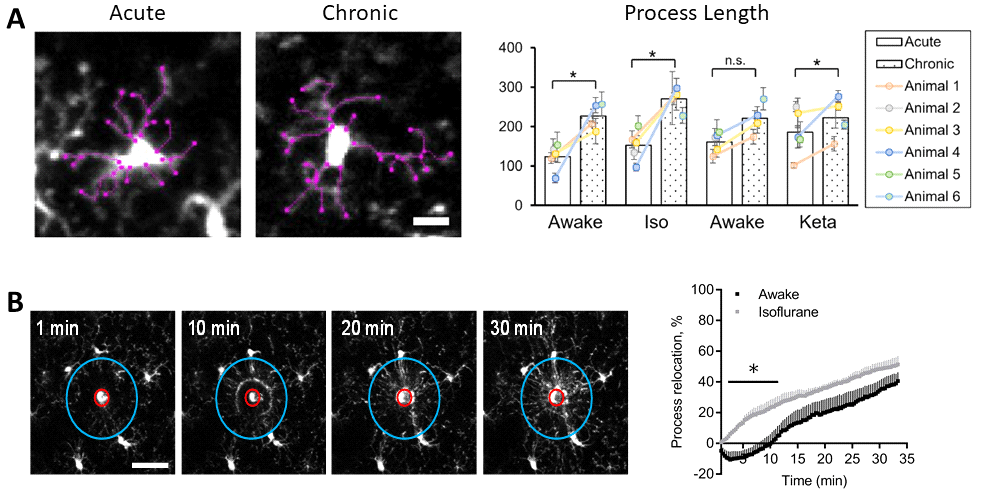
Example readouts of microglia dynamics assays performed at Neurotar. (A) Dynamics of microglial processes in unperturbed conditions. (B) Dynamics of microglial processes after laser-induced lesion, demonstrating that microglia behave differently in awake and anesthetized mice. (A,B) Images were obtained using two-photon microscopy of the somatosensory cortex of awake or anesthetized mice. Figures adapted from Neurotar’s publication.
With two-photon microscopy, we can image brains in live mice at large imaging depths (up to 1 mm) and with high resolution (up to organelle level). In addition, due to low phototoxicity, we can perform live-cell imaging and we can follow the same region in the same mouse for up to several months. Importantly, two-photon imaging allows for reducing group sizes as each animal can serve as its own control. All-in-all, two-photon microscopy is the best method to study microglia in their natural environment: the living brain. To read more about the benefits of two-photon microscopy for in vivo brain imaging, please visit our dedicated web page.
How can Neurotar facilitate your research on microglial dynamics?
Neurotar is the world’s leading commercial service provider of in vivo two-photon brain imaging in mice. As outlined above, two-photon imaging is the preferred technique for imaging microglia in their natural environment: the brain of a living mouse. We have successfully completed multiple microglial dynamics studies for a variety of commercial customers, mostly to assess the efficacy or mode of action of a lead compound. In most studies, we induce microglia activation using laser-induced microlesions. However, we can also activate microglia by other means, for example, topical injection of LPS.
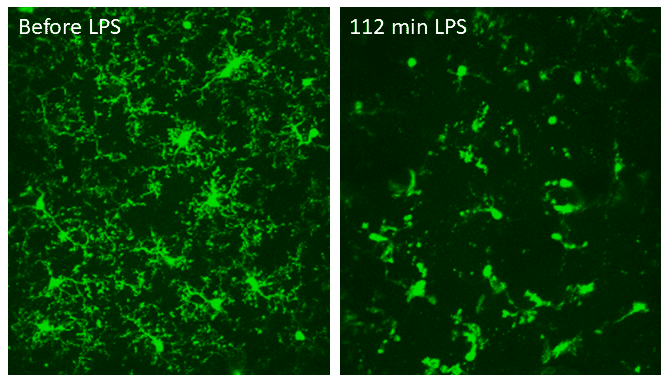
The effect of pro-inflammatory LPS on microglia. Two-photon imaging of microglia in the same region in the somatosensory cortex of a living mouse, before and 112 minutes after topical injection of LPS. LPS caused the microglia to retract most of their processes and round up, consistent with microglia activation. Data obtained at Neurotar.
Possible readouts for these studies include cell morphology (microglia soma size, process branching, process density, dendritic tree area), process motility, and microglial migration. In addition, we can assess the co-localization of fluorescently-tagged molecules (e.g., BBB-penetrating antibodies) with microglia and their sub-cellular organelles (e.g., lysosomes).
At Neurotar, we can image microglia in the brains of awake, behaving mice freely moving in 2D (in vigilo = in awake). This is important since ketamine and other anesthetics can alter microglial function. In vigilo imaging is enabled by our proprietary Mobile HomeCage, which combines stable head fixation with a flat-floored air-lifted cage.
Typical experimental setup for a microglial dynamics assessment
- 2-5 groups, e.g., 1 placebo, 1-2 test compounds or doses, and (optionally) a positive control and/or additional negative control
- 3-5 imaging sessions: baseline at 0 h, 2-4 imaging sessions over several days, weeks, or months
- 3 imaging regions per animal; one region without damage, one region with 3 minor laser-induced damage sites and one region with one major laser-induced damage site (see image below)
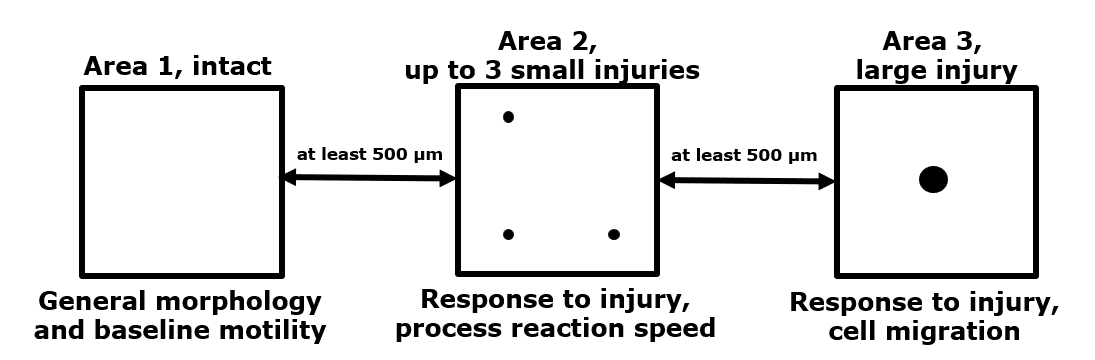
General setup to measure microglial dynamics. Within the same mouse, we can assess unperturbed microglial dynamics, as well as microglial activation induced by two types of laser-induced damage.
Mouse Models
We use transgenic mice with fluorescently labeled microglia. For example, we have used the CX3CR-1GFP mouse line, that expresses GFP in monocytes, dendritic cells, NK cells, and brain microglia under control of the endogenous Cx3cr1 locus. Neurotar can negotiate the commercial license with the licensor on a client’s behalf. Alternatively, clients can extend their license to Neurotar for the duration of the study.
Options and extensions
- Drug administration through a variety of routes, for example, oral (gavage), through i.p., s.c., i.v. or i.t. (intrathecal) injection, or through injection into the Cisterna Magna.
- We can combine experiments with pharmacokinetics.
- In addition to microglial dynamics experiments, we can perform simple behavioral readouts, for example, von Frey nociception assays and hot plate tests.
- We can study microglial dynamics under several brain injury conditions, such as severe global ischemia and focal stroke.
- Additional readouts can be added to the study, for example, quantification of trans blood-brain barrier pharmacokinetics or blood-brain barrier integrity.
- After the experiment has finished, we can harvest and preserve tissues for further analysis.
Other two-photon brain imaging services offered by Neurotar
| Neurodeg. Disease (e.g. AD, PD) | Stroke and TBI | Neuropathic Pain and Migraine | Neuropsychiatry (e.g. Schizophrenia) | Epilepsy | |
|---|---|---|---|---|---|
| Blood-brain barrier integrity | ✓ | ✓ | ✓ | ||
| Trans-BBB pharmacokinetics | ✓ | ✓ | ✓ | ||
| Dendritic spine turnover | ✓ | ✓ | ✓ | ✓ | ✓ |
| Microglial dynamics or response to injury | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium signaling | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abeta Plaque or Tau Tangle dynamics | ✓ | ✓ | ✓ | ||
| Mitochondrial fragmentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ischemic Stroke model | ✓ | ||||
| Regeneration of peripheral neurons | ✓ | ✓ | ✓ |
References
- Sun W, Suzuki M, Toptunov D, Stoyanov D, Yuzaki M, Khiroug L, Dityatev A. (2019) In vivo Two-Photon Imaging of Anesthesia-Specific Alterations in Microglial Surveillance and Photodamage-Directed Motility in Mouse Cortex. Front Neurosci May 7;13:421. DOI: 10.3389/fnins.2019.00421.
- Colonna M, Butovsky O. (2017) Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual Review of Immunology; Vol. 35:441-468. DOI: 10.1146/annurev-immunol-051116-052358.
- Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. (2018) Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci., Sep 27;12:323. DOI: 10.3389/fncel.2018.00323.
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, …, Gross CT. (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun Mar 26;9(1):1228. DOI: 10.1038/s41467-018-03566-5.
- Reshef R, Kudryavitskaya E, Shani-Narkiss H, Isaacson B, Rimmerman N, Mizrahi A, Yirmiya R. (2017). The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb. Elife 6:e30809. DOI: 10.7554/eLife. 30809.
- Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, …, Sierra A. (2020) Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. Journal of Neuroscience 40 (7) 1453-1482; DOI: https://doi.org/10.1523/JNEUROSCI.0993-19.2019.
- Haruwaka K, Ikegami A, Tachibana Y , Ohno N, …, Wake H. (2019) Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun Dec 20;10(1):5816. DOI: 10.1038/s41467-019-13812-z.
- Dudiki T, Meller J, Mahajan G, Liu H, …, Byzova TV. (2020) Microglia control vascular architecture via a TGFβ1 dependent paracrine mechanism linked to tissue mechanics. Nature Commun Feb 20;11(1):986. DOI: 10.1038/s41467-020-14787-y.
- Halder SK, Milner R. (2019) A critical role for microglia in maintaining vascular integrity in the hypoxic spinal cord. Proc Natl Acad Sci Dec 17;116(51):26029-26037. DOI: 10.1073/pnas.1912178116.
- Hansen DV, Hanson JE, Sheng M. (2018) Microglia in Alzheimer’s disease. J Cell Biol. Feb 5; 217(2): 459–472. DOI: 10.1083/jcb.201709069.
- Leng F, Edison P. (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol Mar;17(3):157-172. DOI: 10.1038/s41582-020-00435-y.
- Ferreira SA, Romero-Ramos M, (2018) Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front Cell Neurosci Aug 6;12:247. DOI: 10.3389/fncel.2018.00247.
- Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D’Ambrosi N, The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front Aging Neurosci. 2017; 9: 242. DOI: 10.3389/fnagi.2017.00242.
- Palpagama TH, Waldvogel HJ, Faull RLM, Kwakowsky A. (2019) The Role of Microglia and Astrocytes in Huntington’s Disease. Front Mol Neurosci Oct 25;12:258. DOI: 10.3389/fnmol.2019.00258.
- Mallya AP, Wang HD, Lee HNR, Deutch AY. (2019) Microglial pruning of synapses in the prefrontal cortex during adolescence. Cereb Cortex Apr 1;29(4):1634-1643. DOI: 10.1093/cercor/bhy061.
- Miyamoto A, Wake H, Ishikawa AW, Eto K, …, Nabekura J. (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun Aug 25;7:12540. DOI: 10.1038/ncomms12540.
- Gomez-Nicola D, Perry VH, (2015) Microglial Dynamics and Role in the Healthy and Diseased Brain; A Paradigm of Functional Plasticity. Neuroscientist 2015 Apr; 21(2): 169–184. DOI: 10.1177/1073858414530512.
- Liu YU, Ying Y, Li Y, Eyo UB, Chen T, … Wu LJ. (2020) Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci. 2019 Nov; 22(11): 1771–1781. DOI: 10.1038/s41593-019-0511-3.