High-resolution tests in the brain of awake mice require careful selection of suitable cranial preparations. In vivo cranial preparations vary in terms of cranial window sizes and types of skull modifications. The choice of preparation depends on the research method (imaging or electrophysiology), brain region of interest and length of planned experiments.
To help you find an optimal solution for your project, we have assembled a table comparing various cranial preparations. This table also helps to establish the compatibility of different preparations with the Mobile HomeCage and Mobile HomeCage Large and offers suggestions for the best suitable head plates available from Neurotar.
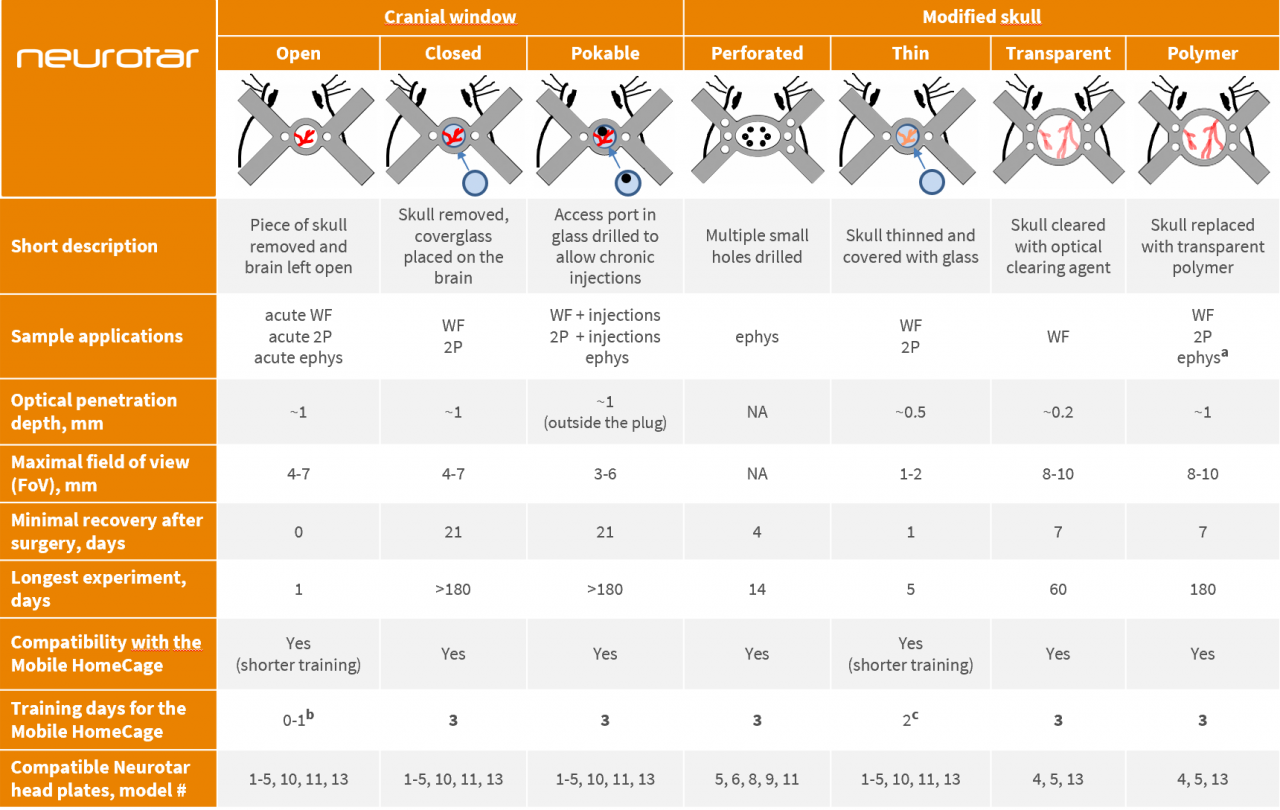
Abbreviations and notes
WF – Wide-field imaging; 2P – two-photon imaging; ephys – electrophysiology
a – for electrophysiological experiments, use soft polymers only.
b – for open cranial window preparations, we recommend either skipping the training or limiting it to a single-day brief training session. Alternatively, you may implant the head plate prior to performing the craniotomy and train mice for the recommended full 3 days before the craniotomy (the gap between head plate implantation and craniotomy may be longer than 4 days, but training should be timed to precede the craniotomy).
c – for thinned skull windows, we recommend shortening the training as window quality starts to degrade several days after the surgery. Alternatively, you may implant the head plate prior to performing the skull thinning surgery and train mice for the recommended full 3 days before the surgery (the gap between head plate implantation and craniotomy may be longer than 3 days, but training should be timed to precede the surgery).
Optical penetration depth and experiment duration estimates are based on recent expert publications and Neurotar’s internal expertise. For details on surgical procedures and experimental protocols please refer to the publications listed below or get in touch with your Neurotar contact.
Original publications
- closed cranial window: Holtmaat A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window, Nat. Protoc., 4 (2009), pp. 1128-1144 doi: 10.1038/nprot.2009.89
- pokable cranial window: Roome C.J. , Kuhn B. Chronic cranial window with access port for repeated cellular manipulations, drug application, and electrophysiology Front. Cell. Neurosci., 8 (2014), p. 379 doi: 10.3389/fncel.2014.00379
- thinned skull: Drew P. et al. Chronic imaging and manipulation of cells and vessels through a polished and reinforced thinned-skull, Nat. Methods., 7 (2010), pp. 981-984 doi: 10.1038/nmeth.1530
- transparent skull: Steinzeig A. et al. Chronic imaging through “transparent skull” in mice, PLoS One., 12 (2017) e0181788 doi: 10.1371/journal.pone.0181788; Zhao Y.-J. et al. Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution, Light Sci Appl ., 7 (2018) p. 17153 doi: 10.1038/lsa.2017.153
- polymer skull: Heo C. et al. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci Rep., 6 (2016), p. 27818. doi: 10.1038/srep27818; Ghanbari L. et al. Cortex-wide neural interfacing via transparent polymer skulls Nat. Commun., 10 (2019), p. 1500 doi: 10.1038/s41467-019-09488-0
Want to learn more about the Mobile HomeCage or get expert advice on selecting a suitable in vivo cranial preparation for your awake experiments?