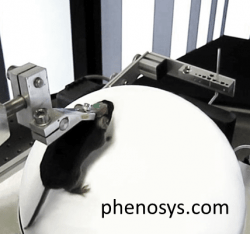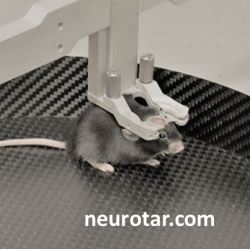
Approaches to studying the brain functions of awake behaving mice
Recording neuronal activity in awake behaving mice is indispensable for studying sensory processing, cognition, or decision making. During an experiment, the targeted brain region must remain stable relative to the optical or electrical probe. This task is not trivial, because an awake mouse wants to move and explore its environment.
In this post, we offer an overview of various approaches to working with awake behaving mice. We consider their advantages and disadvantages and share advice for selecting an optimal approach or a system. If some topics outlined below do not appear relevant, feel free to skip to the appropriate section by clicking on any menu item:
- Head-mounted versus head-fixed alternatives
- Head-mounted approach: an overview
- Head-fixed approach: an overview
- Success factors for experiments with awake behaving mice
- Guide to commercial head fixation systems for awake behaving mice
- Newest DIY head-fixation solutions
- Head-fixed and head-mounted approaches are not mutually exclusive
Head-mounted versus head-fixed alternatives
To achieve the stability of imaging or recording, neuroscientists can do one of two things. They can mount a miniaturized probe on the head of a freely moving mouse (“head-mounted” approach). Alternatively, they can immobilize the animal’s head in a stationary frame for stable access with full-size probes and advanced optical equipment, such as a two-photon microscope (“head-fixed” or “head-restrained” approach).
Head-mounted approach: an overview
Electrophysiologists have used the head-mounted solutions since the 1970s. More recently, miniaturized optical microscopes were developed (Ghosh et al./Schnitzer, 2011). Such microscopes have since become widely available both as commercial products (e.g., nVista and nVoke from Inscopix Inc, www.inscopix.com, and Quartet from Neurescence Inc., www.neurescence.com) and as do-it-yourself (DIY) solutions (UCLA’s Miniscope, www.miniscope.org; University of Toronto’s CHEndoscope).
Mice carrying a head-mounted device can move in a three-dimensional environment. However, their movement is constrained by the weight and length of the attached cables. Moreover, the performance of a head-mounted microscope is compromised by miniaturization. This approach works best for studying neuronal networks (with relatively low resolution) within a limited field of view of a pre-implanted endoscopic probe.
Head-fixed approach an overview
In the head-fixed camp, the research has progressed from anesthetized or fully-restrained mice to head-fixed awake and behaving mice. To minimize stress caused by immobility, researchers place awake head-fixed mice on a linear or circular treadmill, on an air-lifted ball, or in a floating flat-floored cage (Dombeck et al./Tank 2007, Royer et al./Buzsaki 2012, Kislin et al./Khiroug 2014). Solutions that combine head-fixation with body movement are available both as DIY (Dombeck and Tank, 2017; Nashaat et al./Larkum, 2016; Voigts et.al./Harnett, 2018) and as commercial solutions outlined below.
In head-fixed mice, researchers can study brain activity at a cellular or subcellular level using high-resolution multi-color microscopy methods or single-cell electrophysiology. However, head-fixed mice move in only one or two dimensions, their natural head movements are restricted, and vestibular inputs are compromised (Thurley and Ayaz, 2016).
Success factors for experiments with awake behaving mice
What factors should you consider while choosing an optimal head-fixation solution?
Mechanical stability
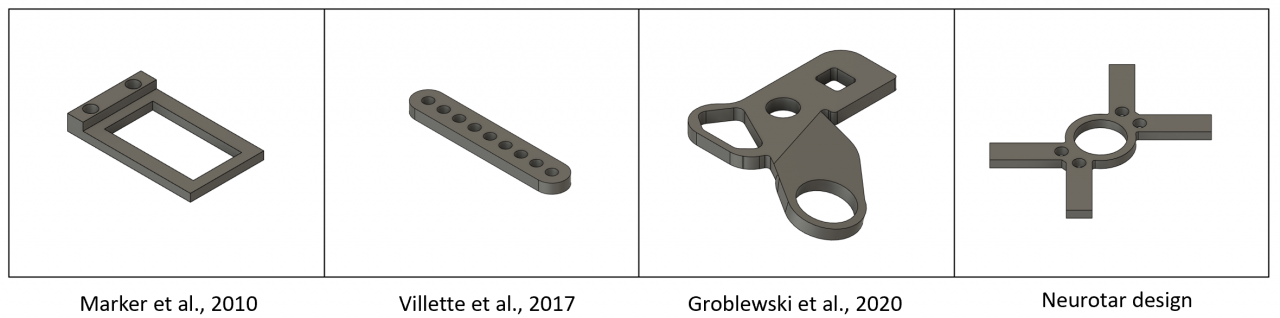
The main reason for head-fixation is achieving imaging and recording stability. The mechanical stability is a factor of the overall system design, but especially of the head-fixation apparatus’ design. Such apparatus usually consists of the head plates (a.k.a. head bars or head holders) and a clamping mechanism. The latter should be robust, yet not bulky as not to obstruct the mouse’s vision. If you need to access lateral parts of the brain, the head fixation must rotate. Ideally, it should also be adjustable in the nodding direction. Last but not least, the head-fixation should be fast as the head-fixation process is inherently stressful to the mouse. Whenever possible, the head plate should have multiple fixation points to prevent its movement during the experiment. Below are the pictures of some common head plate designs. At Neurotar, we have developed a variety of head plate options with four blades for extra stability.
Low-stress environment
Mechanical stability is only one part of the story. Imaging and recording stability also depends on the level of stress experienced by the mouse during the experiment. The stress resulting from inability to move the head is further compounded, if the mouse feels exposed, lacks the natural sensory stimulation, or does not control its limbs’ movements. Stressed mice make jerky movements trying to free themselves from head-fixation. In addition to visual signs of stress, the mouse’s brain chemistry and activity are affected. It is possible to mitigate some of this stress with training. All the same, we recommend choosing a solution that closely mimics the mouse’s familiar dark and enclosed cage environment.
Quality of surgery
Invest the time upfront in acquiring or improving surgical skills. Ensure that there is no residual dental cement left on the head plate after surgery as this may compromise imaging and recording stability. Closely monitor the mouse’s recovery and discard the animals that show the signs of discomfort or inflammation. We recommend housing the operated mice in group cages to facilitate their well-being and lower the stress. When the surgeries are done well, the head plates do not come off, and the mice do not damage their own or other mice’ cranial windows. At Neurotar, we recognize the importance of surgeries’ quality. We share online surgery tutorials with our customer labs. If you opt for another solution, ask your supplier or the labs that use their equipment for help with the surgery.
Adequate mouse training
Last but not least, invest in acclimating, habituating, and training mice before head-fixing them for imaging or recording experiments. There is no skipping steps in this process. But the solutions that closely mimic the cage environment and thus minimize the mouse’s stress during head-fixation, allow reducing the required amount of training. Opt for shorter but more frequent training sessions and allow time in between the sessions for memory consolidation. Whenever possible, avoid using water restriction or transient anesthesia during training. Neither is a prerequisite for an adequately habituated and trained mouse and a well-designed head-fixation system: we never use anesthetics or water deprivation while training mice for the Mobile HomeCage.
Guide to commercial head fixation systems for awake behaving mice
If you must head-fix a mouse for your experiments, there are various commercial and DIY options to choose from. They range between full-body restraint and linear one-dimensional or two-dimensional treadmills with various degrees of behavioral integration. Two-dimensional treadmills come in two types: spherical treadmills and flat-floored solutions. Your experimental design ultimately determines the choice. These are some factors to consider:
Below, we have compiled a table of the most common commercial head-fixation solutions. The table allows comparing them alongside the factors outlined above and several others.
Commercial head-fixation solutions (comparative table)
| Type | Linear treadmill | Spherical treadmill + VR | Rotating disc | Flat-floored 2D "treadmill" |
|---|---|---|---|---|
| Supplier | LN Treadmill - Luigs & Neumann | JetBall + VR - Phenosys GmbH | Gramophone - Femtonics Ltd | Mobile HomeCage - Neurotar Oy Ltd |
| Movement dimensions | 1D | 2D | 1D | 2D |
| Free trajectory | No | Yes | No | Yes |
| Locomotion tracking | No | Yes | Yes | Yes |
| Vertically compact | Yes | No | Yes | Yes |
| Tangible cage environment | No | No | No | Yes |
| Requires VR | No | Yes | No | Yes |
| 2D maze navigation | No | Yes, virtual mazes | No | Yes, tangible mazes |
| Social interactions | No | No | No | Yes |
| Validated for spinal cord imaging & recordings | No | No | No | Yes |
| Validated for patch clamp | Yes | No | No | Yes |
| System-specific head plates | No | Yes | Yes | Yes, 13 models |
| Software | Yes | Yes | Yes | Yes, open-source |
| Price range | ~10 K USD (hardware only) | ~50 K USD (JetBall + VR) | ~15 K USD | 18-28 K USD (depending on the model) |
Newest Do-It-Yourself (DIY) solutions for awake behaving mice
Imitation is the best sign of success: two recent innovative DIY solutions draw inspiration from the Mobile HomeCage’s flat-floored design. They are the AirTrack system developed by Matthew Larkum’s lab (Humboldt University) and the rotational head-fixation system developed by Mark Harnett’s lab (MIT).
In its current incarnation, the AirTrack is essentially a behavioral device, not yet validated for stable imaging and recording. The design reproduces an X-maze integrated with operant conditioning. Due to the large footprint and the tracking device’s location underneath the maze, the system is not easily compatible with most commercial 2-photon microscopes.
Harnett lab’s rotational head-fixation system enables the mouse’s head rotation in the xy-plane. This is a significant achievement, which mitigates the lack of vestibular input characteristic of all head-fixation systems. Unfortunately, the head movement remains limited to the xy-plane alone, and the complexity of the design makes it hard to reproduce for the labs without strong engineering capabilities and resources.
Last but not least, we would like to highlight Harvey lab Mouse VR DIY solution, which incorporates the Styrofoam ball and comes complete with the VR, the reward system, and the lick sensor. The solution is freely available on GitHub. It is compatible with the standard 19’’ server rack and allows integrating up to 3 rigs per rack! It incorporates an innovative VR design that relies on the use of a small laser projector. The 3D printing and plastic treatment instructions ensure printed parts’ rigidity and durability. The design includes the “behavior PCB” circuitry (based on Teensy 3.2) complete with lick sensor, ball position reading, and communication with the external hardware (i.e. NI board). In short, if you are thinking about implementing a ball-based solution, think no more! The Harvey lab has already done the work for you.
No research tool is perfect. But, compared to just a few years ago, there is currently a wide variety of commercial & DIY head-fixation solutions to accommodate multiple research needs. If your choice falls on our solution, learn more about the Mobile HomeCage on our website or contact us for help.
Head-mounted and head-fixed approaches are not mutually exclusive
An increasing number of labs are starting to combine them to benefit from the strengths inherent in each approach. Recognizing this trend, Inscopix has recently joined forces with several multiphoton microscope manufacturers in launching a new Multimodal Image Registration and Analysis (MIRA) platform, which allows integrating one-photon and multiphoton imaging.
The current solution has some limitations with regard to the objective’s numeric aperture (NA), which must be around 0.5 to match the numeric aperture of the GRIN lens. The laser power must be increased to compensate for the resulting degradation in image quality. Besides that: the working distance must be between 5-10 mm. Nonetheless, this is a promising new step toward combining the head-mounted and head-fixed approaches.
Contact us using for a free consultation on how to combine various approaches or selecting the one that best suits your experimental needs.