
Two-photon imaging of calcium signaling in the brain of a living mouse. Image obtained at Neurotar.
Calcium imaging in awake, behaving mice
We measure calcium signaling, a proxy for neuronal activity, in the most physiologically relevant model system: the brains of awake, behaving mice. Our assays are uniquely suited to longitudinally assess drug efficacy or mode of action (MoA), or to characterize mouse disease models:
- We can measure the activity of individual neurons in a designated brain region using two-photon microscopy.
- We can measure calcium signaling across the entire cortex for analysis of network connectivity in health and brain disease using our very own Invigilo wide-field imaging system optimized for experiments in awake mice.
Calcium signaling as a proxy for neuronal activity
In neurons under resting conditions, Ca2+ concentrations in the cytoplasm are much lower than in the endoplasmic reticulum, mitochondria, and extracellular space. Neuronal excitation by an action potential causes calcium channels to open, which leads to a massive influx of Ca2+ ions into the cytoplasm. This triggers many downstream events, including gene transcription, neurotransmitter release, neurite outgrowth, and the activation of calcium-dependent enzymes. After the action potential has peaked, Ca2+ levels return to normal very quickly. Because of this strong correlation between neuronal activity and fluctuations in intracellular Ca2+ levels, calcium signaling measurements have become a gold standard to assess neuronal activity. In addition, calcium signaling measurements can be used to identify and study neuronal networks.
Calcium imaging in the intact brain
The development and optimization of calcium indicators have greatly facilitated calcium imaging studies. A frequently used calcium indicator is the genetically encoded GCaMP, which very rapidly switches back and forth between a non-fluorescent and a brightly fluorescent state in response to changing Ca2+ levels. This makes GCaMP an ideal solution to measure fluctuations in Ca2+ levels.
Calcium signaling can be imaged in a variety of in vitro and ex vivo preparations, such as cultured neuronal cells or acute brain slices. While generating important insights, such preparations do not capture the complexity of an intact brain in a living organism. Therefore, the relevance of data obtained using such assays is limited. Fortunately, transgenic mice expressing GCaMP in excitatory neurons in the brain enable calcium imaging in live animals.
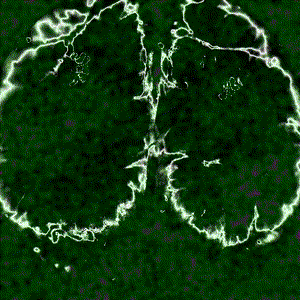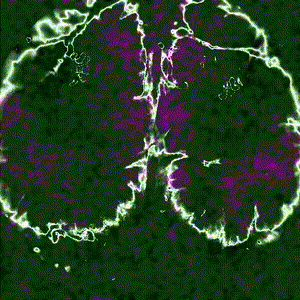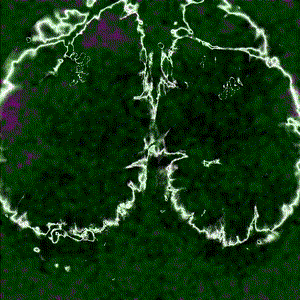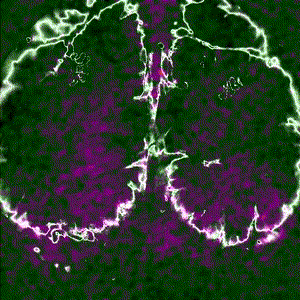
Cortex-wide calcium signaling (dF/F) in a living, behaving mouse expressing GCaMP in excitatory neurons. Image obtained at Neurotar using wide-field calcium imaging on the Invigilo setup.
Why use two-photon microscopy to study calcium signaling?
With two-photon microscopy, we can image brains in live mice at large imaging depths (up to 1 mm) and with a high spatial resolution (organelle level). In addition, due to low phototoxicity, we can perform live imaging with high temporal resolution (below 100 ms) and we can follow the same region in the same mouse for up to several months. To read more about the benefits of two-photon microscopy for in vivo brain imaging, please visit our dedicated web page.
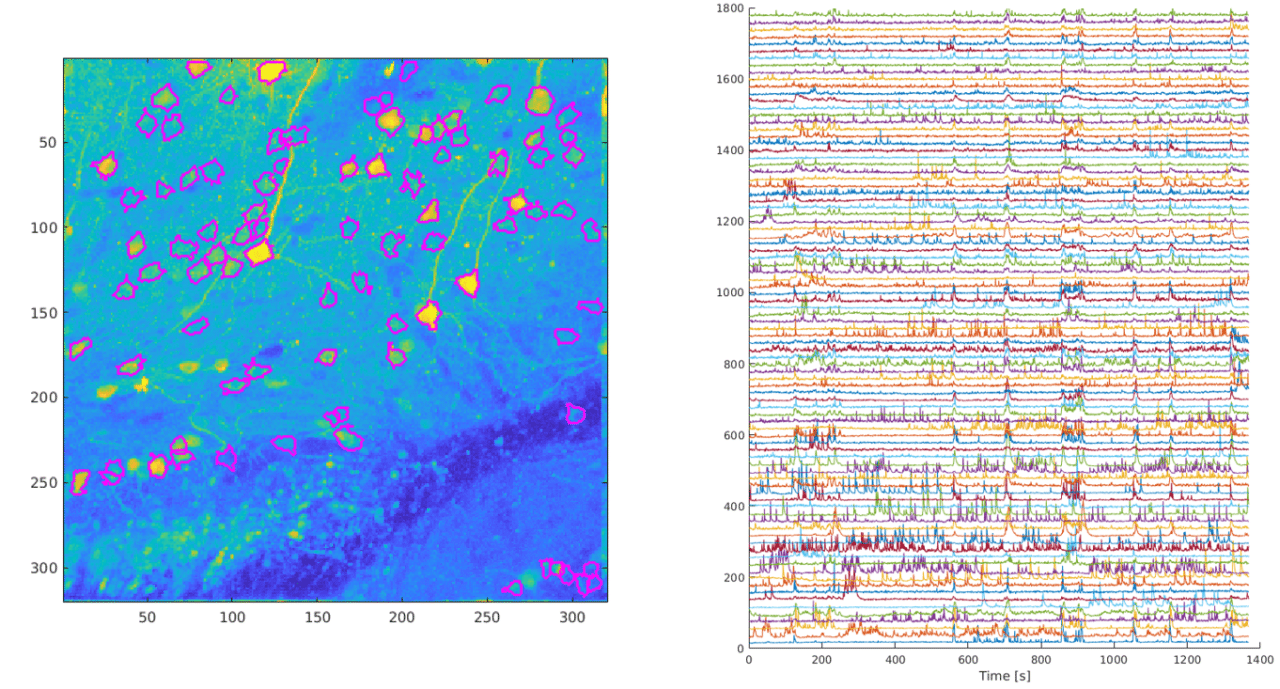
The activity of individual neurons can be tracked over extended periods of time when calcium signaling is imaged using two-photon microscopy. Data obtained at Neurotar.
Why use wide-field imaging to study calcium signaling?
The brain functions through coordinated activity between brain regions. Offering a large field of view and high temporal resolution (< 100 ms), wide-field microscopy enables visualization and quantification of network connectivity across the entire cortex. Therefore, wide-field calcium imaging is an excellent tool to assess the efficacy or MoA of compounds that are expected to affect such networks. Examples of such compounds include drugs for a variety of brain disorders (such as schizophrenia), but also psychedelics.
How can Neurotar facilitate your research using calcium signaling?
Neurotar is the world’s leading commercial service provider of in vivo brain imaging in mice. We have done calcium signaling studies for a variety of commercial customers. Importantly, calcium signaling studies at Neurotar can be performed in awake, behaving mice using our proprietary Mobile HomeCage and Invigilo. This is highly relevant as ketamine and other general anesthetics strongly affect calcium signaling.
In a typical two-photon calcium signaling assay, we image neuronal activity over time in a certain brain region. Due to low phototoxicity and the ability to obtain high-resolution images, our two-photon microscopy calcium imaging assay enables us to track the activity of individual neurons over extended periods of time. This provides a wealth of data, as illustrated above, which increases statistical power. In addition, we can re-examine the same field of view at different time points over several months. This enables us to measure neuronal activity in the same neurons before and after, for example, a perturbation (illustrated below for spinal cord injury) or drug treatment. Measuring control and treatment conditions on the same neurons decreases variability and reduces the number of animals needed for each study.
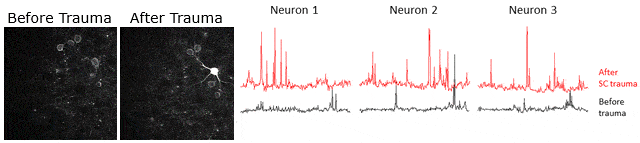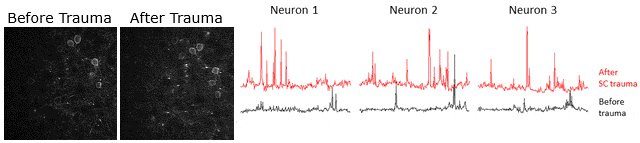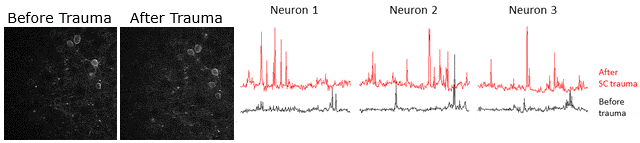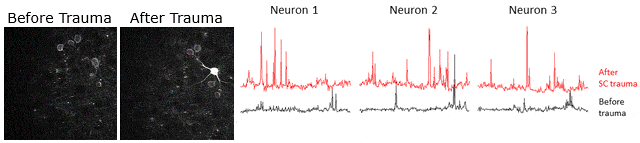
Example of calcium signaling data obtained using two-photon microscopy at Neurotar. Calcium signaling was imaged (videos in left panels) and quantified (3 right panels) in the same neurons before and after spinal cord injury, showing that spinal cord injury causes an increase in neuronal activity.
A typical widefield calcium imaging study records cross-cortical neuronal activity for extended periods of time. This can be done before and at different time points after treatment with a lead compound, for example, to assess efficacy or MoA. Alternatively, mouse disease models can be characterized through comparison with WT mice of the same genetic background.
Typical experimental setup
- 2-5 groups, e.g., 1 placebo, 1-2 test compounds, and potentially a positive control and/or additional negative control
- 3-5 imaging sessions: baseline at 0 h, 2-4 imaging sessions over several hours, days, weeks, or months
Mouse Models
In most studies, we use transgenic mice that express GCaMP in excitatory neurons, for example, mouse line C57BL/6J-Tg(Thy1-GCaMP6f)GP5.5Dkim/J . However, we can also use most other transgenic mouse models. This includes commonly used models for various neurological diseases such as Alzheimer’s (for example P301S and APP/PS1) and Parkinson’s disease (for example LRRK2). Viral vectors such as pAAV.Syn.GCaMP6f.WPRE.SV40 can be used to drive neuronal GCaMP expression in such disease models. Using viral vectors allows reducing study cost (wild-type mice are cheaper than recombinant mice) and duration, as well as using aged mice without a delay. Neurotar can acquire a commercial license on a client’s behalf. Alternatively, clients can extend their license to Neurotar for the duration of the study.
Options and extensions
- Drug administration through a variety of routes, for example, oral (gavage), through i.p., s.c., i.v. or i.t. (intrathecal) injection, or injection into the Cisterna Magna.
- In addition to calcium imaging, we can track animal movement or perform simple behavioral readouts, for example, von Frey nociception assays and hot plate tests.
- We can install a micro prism for “lateral” imaging of cortical layers at 90 degrees
- Additional readouts can be added to the study, for example, quantification of Abeta plaques, Tau tangles, blood flow, pharmacokinetics, or blood-brain barrier integrity.
- We can measure calcium signaling under several CNS injury conditions, such as severe global ischemia, focal stroke, and spinal cord injury.
- We can identify and study neuronal networks from the calcium signaling data.
- At the end of the study, we can harvest and preserve tissues for further analysis.
Other two-photon brain imaging services offered by Neurotar
| Neurodeg. Disease (e.g. AD, PD) | Stroke and TBI | Neuropathic Pain and Migraine | Neuropsychiatry (e.g. Schizophrenia) | Epilepsy | |
|---|---|---|---|---|---|
| Blood-brain barrier integrity | ✓ | ✓ | ✓ | ||
| Trans-BBB pharmacokinetics | ✓ | ✓ | ✓ | ||
| Dendritic spine turnover | ✓ | ✓ | ✓ | ✓ | ✓ |
| Microglial dynamics or response to injury | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium signaling | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abeta Plaque or Tau Tangle dynamics | ✓ | ✓ | ✓ | ||
| Mitochondrial fragmentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ischemic Stroke model | ✓ | ||||
| Regeneration of peripheral neurons | ✓ | ✓ | ✓ |
References
- Grienberger C, Giovannucci A, Zeiger W, Pontera-Cailliau s. (2022) Two-Photon Calcium Imaging of Neuronal Activity. Nature Reviews Methods Primers 2, 67. DOI: 10.1038/s43586-022-00147-1
- Kawamoto EM Vivar C, Camandola S. (2012) Physiology and Pathology of Calcium Signaling in the Brain. Front Pharmacol. 3: 61. DOI: 10.3389/fphar.2012.00061.
- Nakai J, Ohkura M, Imoto K. (2001) A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 19(2):137-41. DOI: 10.1038/84397.
- Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, …, Looger LL. (2012) Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 32(40):13819-40. DOI: 10.1523/JNEUROSCI.2601-12.2012.
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, …, Svoboda K, Kim DS. (2013) Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature. 499(7458): 295–300. DOI: 10.1038/nature12354.
- Cramer JV, Gesierich B, Roth S, Dichgans M, Düring M, Liesz A. (2020) In vivo widefield calcium imaging of the mouse cortex for analysis of network connectivity in health and brain disease. Neuroimage. 199:570-584. DOI: 10.1016/j.neuroimage.2019.06.014.
- Hamm JP, Peterka DS, Gogos JA, Yuste R. (2017) Altered cortical ensembles in mouse models of schizophrenia. Neuron. 94(1): 153–167.e8. DOI: 10.1016/j.neuron.2017.03.019.
- Seshadri S, Hoeppner DJ, Tajinda K. (2020) Calcium Imaging in Drug Discovery for Psychiatric Disorders. Front Psychiatry. 11: 713. DOI: 10.3389/fpsyt.2020.00713.
- Lu J, Tjia M, Mullen B, Cao B, Lukasiewicz K, Zuo Y. (2021) An analog of psychedelics restores functional neural circuits disrupted by unpredictable stress. Molecular Psychiatry volume 26, pages6237–6252. DOI: 10.1038/s41380-021-01159-1.
- Lisek M, Zylinska L, Boczek T. (2020) Ketamine and Calcium Signaling-A Crosstalk for Neuronal Physiology and Pathology. Int J Mol Sci. 21(21):8410. DOI: 10.3390/ijms21218410.
- Wang HY, Eguchi K, Yamashita T, Takahashi T. (2020) Frequency-Dependent Block of Excitatory Neurotransmission by Isoflurane via Dual Presynaptic Mechanisms. J Neurosci. 40 (21) 4103-4115. DOI: https://doi.org/10.1523/JNEUROSCI.2946-19.2020.