Two-photon imaging in a nutshell
Several microscopy techniques exist to image in vitro or ex vivo samples, such as cultured cells and tissue slices. A limit of such ‘one-photon’ microscopy is that the light used to excite most common fluorophores (fluorescent molecules) is very effectively absorbed by living tissue. As a result, this light does not penetrate very far, which prevents imaging of tissue in a living animal.

Two-photon microscopy overcomes these limitations. The low-energy infra-red light used for excitation penetrates deep into living tissue. Therefore, with two-photon imaging we can acquire images at great imaging depth (up to 1 mm) and with high-resolution (down to organelle level). Importantly, two-photon microscopy works with the same common fluorophores as used for conventional ‘one-photon’ techniques.
In addition to penetrating deep into tissue, infra-red light causes minimal damage. This enables repeated imaging of the exact same region in the same animal for up to several months, providing a wealth of information. Furthermore, we can use each animal as its own control, which reduces variability and allows smaller group sizes. For these reasons, two-photon imaging adheres to the 3R principle for animal research (Refine, Reduce, Replace). All-in-all, two-photon microscopy is the ideal solution to measure drug efficacy and disease progression over time in the most relevant environment: a living animal.
The brain is especially suited for two-photon microscopy. We can image several regions in the mouse brain, such as the prefrontal and somatosensory cortex, with high resolution, at great depth, and with low phototoxicity. This enables for example measurement of blood-brain barrier (BBB) integrity, trans-BBB pharmacokinetics, dendritic spine turnover, microglial dynamics, Abeta plaque and Tau tangle dynamics, calcium signaling, and mitochondrial fragmentation.
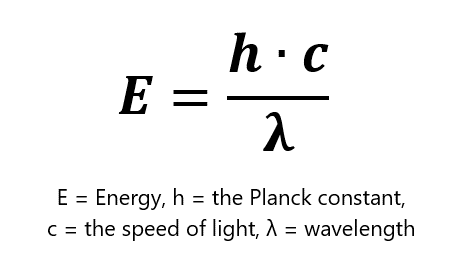
Two-photon microscopy in technical terms
For fluorescence to occur, a fluorophore needs to absorb a photon with a specific energy to become excited, and subsequentially emit a photon of lower energy. Most commonly used fluorophores absorb visual light of low wavelength. Such light is very effectively absorbed by tissue, which limits how far the light can penetrate. Therefore, low wavelength light is not suited to image intact tissue. Infra-red light has much better tissue penetration, but one infra-red photon does not pack enough energy to excite those common fluorophores. After all, the energy of a photon inversely correlates with the photon’s wavelength.
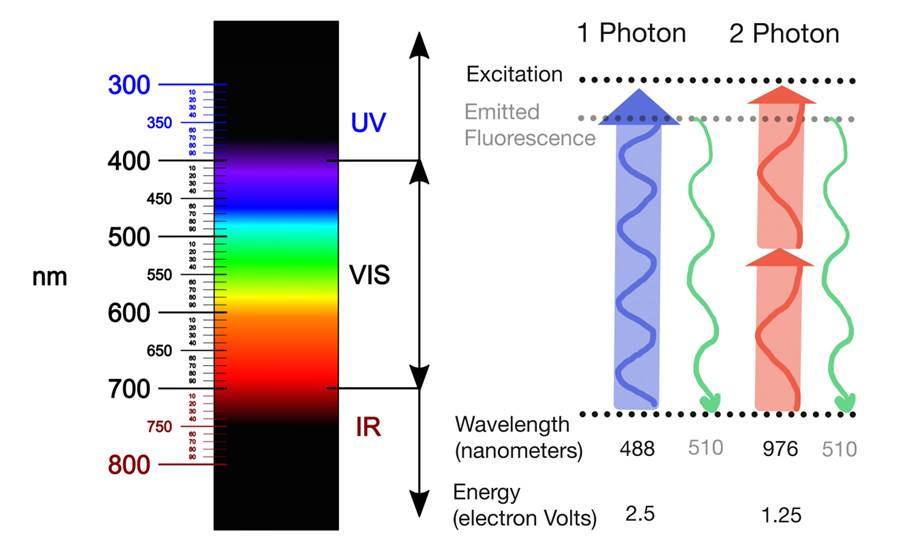
Two-photon microscopy relies on the ability of two simultaneously-absorbed infra-red photons to excite such common fluorophores. Given that E = hc/λ, the wavelength of those infrared photons should be twice as high as the wavelength for one-photon excitation. The fluorophore emits only one photon, however, which is of the same wavelength regardless of whether it was excited with one- or two-photon excitation. Therefore, two-photon microscopy does not require any special detectors.
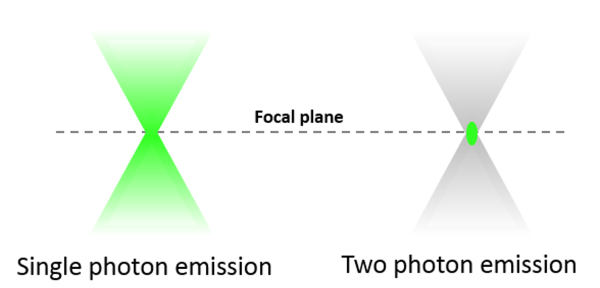
One important advantage of two-photon microscopy is that excitation (and thus emission) is confined to a small volume around the focal point. This is because only in this region photon flux is sufficient to give rise to two-photon absorption events. Spatial confinement of two-photon excitation provides several advantages over single-photon confocal microscopy. Due to reduced overall photobleaching and photodamage, we can image biological specimens long-term without loss of viability. Secondly, two-photon microscopes collect fluorescence more efficiently than confocal microscopes as they do not require a pinhole. Finally, very localized (within 1 μm in each direction), high-intensity two-photon excitation enables several types of experiments, for example induction of neural injury or stroke, or release of caged compounds to measure diffusion.
For two-photon excitation to occur, the fluorophore has to absorb two photons within ~10−18 sec. This requires a very high photon flux. Therefore, one disadvantage of two-photon microscopy is that it requires much higher laser power, and thus a much more powerful and expensive laser, than one-photon excitation.
How can Neurotar assist you in your research?
Neurotar is the world’s leading commercial service provider of in vivo two-photon brain imaging in mice. We offer a broad portfolio of services (follow the links for more information):
Two-photon image of Layer V neurons of the cortex in Thy1-YFP mouse