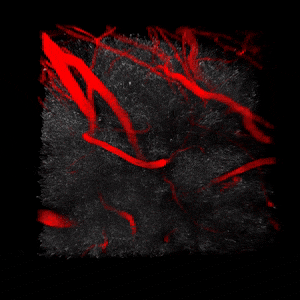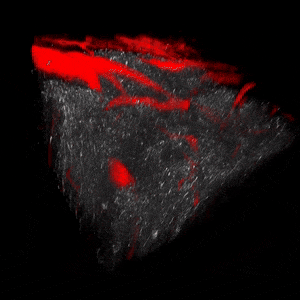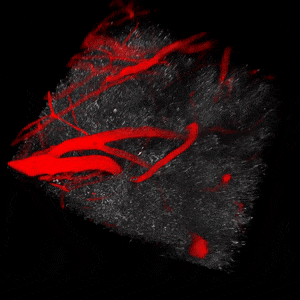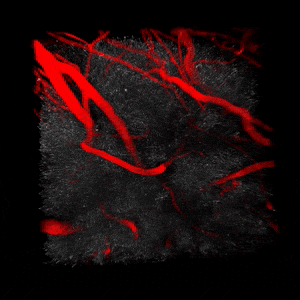
We use two-photon microscopy to study mitochondrial morphology and function as they change over time during disease progression or in response to treatment. We observe mitochondria’s evolution over weeks to months within the physiologically relevant environment of a living mouse brain.
(On the left) 3D reconstruction of CFP-labeled neuronal mitochondria (grey) and vasculature (red) in the brain of a living mouse. 2-photon data obtained at Neurotar.
Mitochondria are every neuron’s best friend… until they are not
Mitochondria are intracellular organelles that support neurons in multiple ways from energy production to calcium regulation, antioxidant defense, and growth. Disruptions in mitochondrial function play a crucial role in acute neurotoxicity in traumatic brain injury and ischemic stroke. Besides that: they accompany slower-developing processes such as neurodegeneration associated with Alzheimer’s, Parkinson’s disease, ALS, and aging.
Abnormally functioning mitochondria generate excessive amounts of toxic reactive oxygen species, lose the ability to buffer intracellular calcium, and eventually initiate the irreversible cascade of events leading to apoptosis and neuronal cell death.
Why study mitochondria using two-photon microscopy?
In vivo two-photon microscopy is a sophisticated imaging technique that offers a combination of depth, precision, and reduced phototoxicity unmatched by traditional microscopy techniques. In vivo two-photon imaging allows observing mitochondrial behavior and dynamics in real-time, within a living brain. This enhances our understanding of how mitochondria respond to various physiological conditions and treatments.
In vitro and ex vivo methods often involve isolating cells or tissues, which can alter mitochondrial behavior. The combination of state-of-the-art labeling techniques with two-photon microscopy allows us to study mitochondrial morphology and function in vivo in an unperturbed physiological environment. Thus, we avoid the detrimental effects of tissue fixation and low oxygenation associated with in vitro and ex vivo techniques (Kim et al., 2022).
Besides revealing where mitochondria are and how they move, in vivo two-photon imaging also sheds light on their functional status.
Finally, in vivo two-photon microscopy allows for longitudinal studies of mitochondrial dynamics over time within the same mouse. This provides a deeper understanding of mitochondrial roles in aging, disease progression, and response to therapies.
What studies can Neurotar perform to elucidate changes in mitochondrial morphology and function?
Visualizing mitochondria using in vivo two-photon microscopy relies on labeling. Several approaches can be utilized for this.
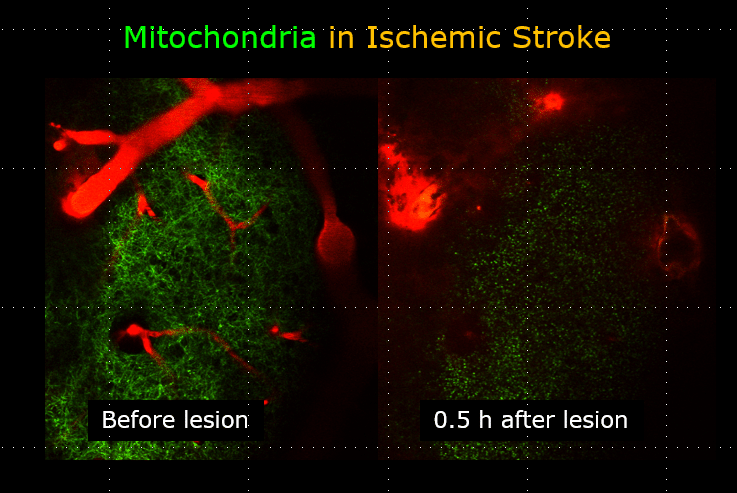
In the past, Neurotar relied on transgenic mouse models that express fluorescent proteins localized to the mitochondria by a human cytochrome c oxidase. For example, the Thy1-CFP-MitoS mouse strain is as an excellent tool for studying mitochondria morphology in the cortical motor neurons. Since mitochondria function is closely linked to its structural properties, studying the transition from tubular to round, vesicular-like shapes allows us to detect the switch between “neuroprotective” to “neurotoxic” forms of mitochondria (Lihavainen et al., 2015; Kislin et al., 2017). This is particularly important for evaluating neuroprotective drug candidates in neurodegeneration, traumatic brain injury, and stroke models.
The image above illustrates changes in mitochondrial morphology after ischemic stroke induction observed using two-photon imaging of CFP-labeled neuronal mitochondria (green) and vasculature (red). Data obtained at Neurotar.
In addition to commercially available tg mouse strains, we are happy to utilize our clients’ in-house mouse models.
New avenues of research into mitochondrial dysfunction
Alternative approaches directly measure mitochondrial oxidative stress or calcium concentration in vivo using viral AAV-driven expression of sensitive fluorescent proteins (Calvo-Rodriguez et al., 2021; Calvo-Rodriguez et al., 2024). These studies aim to prevent the excessive increase in mitochondrial calcium concentration and redox production as a means of delaying neuronal cell death. While our team has not yet had a chance to utilize viral vectors for mitochondrial studies, we are open to trying this approach.
Finally, yet another promising approach targets mitophagy-related changes in mitochondria fluorescence. This is important for identifying treatment options for neurodegenerative conditions such as Parkinson’s disease and for rare mitochondrial diseases (Luo et al., 2021).
Thanks in part to longevity research focusing on mitochondrial dysfunction (Protasoni et al., 2023) and the growing recognition of mitochondria’s crucial role in multiple neurodegenerative diseases, mitochondria are back in the focus of the neuroscience community. Although Neurotar’s studies of mitochondrial dysfunction up to date have focused on stroke and TBI, we welcome the chance to explore these new avenues of research.
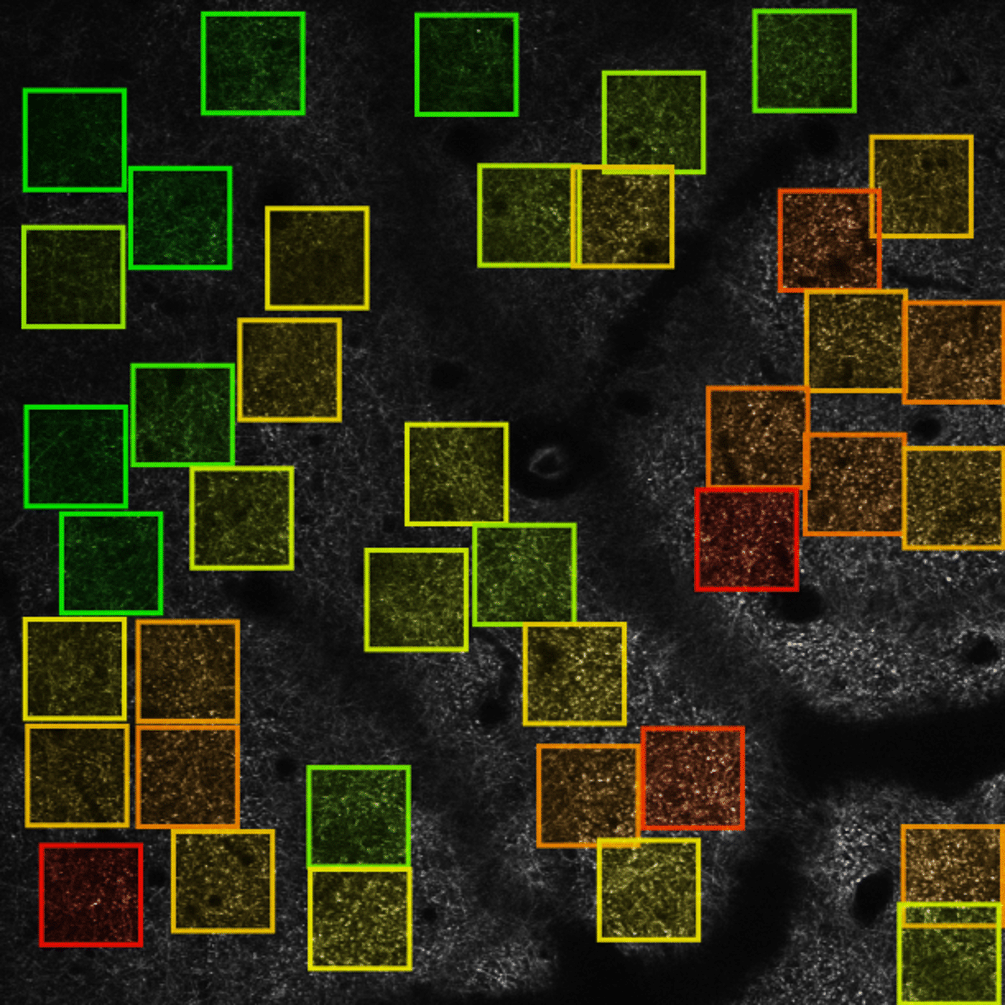
The image on the left illustrates the automated analysis of mitochondria shape after ischemic stroke. Lihavainen et al., 2015. Data obtained at Neurotar.
How can Neurotar facilitate your in vivo studies on mitochondrial dysfunction?
First, we’ll sit down with you to discuss your research questions. Together, we will formulate the hypothesis and design an optimally powered in vivo microscopy study for testing your hypothesis.
Unless you already have access to a mitochondria mouse line, we will help you source the mice from a validated provider of mouse models such as Thy1-CFP-MitoS or AAV viral vector.
Finally, upon completion of the study, we shall present the results and propose our interpretation of the data. We shall then answer all your questions and finalize the report based on your comments and requests. If you’d like to publish some or all of the study results, we’ll be delighted to assist you in writing the Materials and Methods section.
Neurotar’s publications:
Kislin M, Sword J, Fomitcheva IV, Croom D, Pryazhnikov E, Lihavainen E, Toptunov D, Rauvala H, Ribeiro AS, Khiroug L, Kirov SA. (2017) Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J Neurosci. 37(2):333-348. https://doi.org/10.1523/JNEUROSCI.1510-16.2016
Lihavainen E, Kislin M, Toptunov D, Khiroug L, Ribeiro AS. (2015) Automatic quantification of mitochondrial fragmentation from two-photon microscope images of mouse brain tissue. J Microsc. 260(3):338-51. https://doi.org/10.1111/jmi.12301
Cited works:
Calvo-Rodriguez M, Kharitonova EK, Bacskai BJ. (2021) In vivo brain imaging of mitochondrial Ca2+ in neurodegenerative diseases with multiphoton microscopy. Biochim Biophys Acta Mol Cell Res. 1868(6):118998. https://doi.org/10.1016/j.bbamcr.2021.118998
Calvo-Rodriguez M, Kharitonova EK, Snyder AC, Hou SS, Sanchez-Mico MV, Das S, Fan Z, Shirani H, Nilsson KPR, Serrano-Pozo A, Bacskai BJ. (2024) Real-time imaging of mitochondrial redox reveals increased mitochondrial oxidative stress associated with amyloid β aggregates in vivo in a mouse model of Alzheimer’s disease. Mol Neurodegener. 19(1):6. https://doi.org/10.1186/s13024-024-00702-2
Kim SY, Strucinska K, Osei B, Han K, Kwon SK, Lewis TL Jr. (2022) Neuronal mitochondrial morphology is significantly affected by both fixative and oxygen level during perfusion. Front Mol Neurosci. 15:1042616. https://doi.org/10.3389/fnmol.2022.1042616
Luo H, Krigman J, Zhang R, Yang M, Sun N. (2021) Pharmacological inhibition of USP30 activates tissue-specific mitophagy. Acta Physiol (Oxf). 232(3):e13666. https://doi.org/10.1111/apha.13666
Protasoni M, Serrano M. (2023) Targeting Mitochondria to Control Ageing and Senescence. Pharmaceutics. 15(2):352. https://doi.org/10.3390/pharmaceutics15020352
Other two-photon brain imaging services offered by Neurotar
| Neurodeg. Disease (e.g. AD, PD) | Stroke and TBI | Neuropathic Pain and Migraine | Neuropsychiatry (e.g. Schizophrenia) | Epilepsy | |
|---|---|---|---|---|---|
| Blood-brain barrier integrity | ✓ | ✓ | ✓ | ||
| Trans-BBB pharmacokinetics | ✓ | ✓ | ✓ | ||
| Dendritic spine turnover | ✓ | ✓ | ✓ | ✓ | ✓ |
| Microglial dynamics or response to injury | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium signaling | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abeta Plaque or Tau Tangle dynamics | ✓ | ✓ | ✓ | ||
| Mitochondrial fragmentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ischemic Stroke model | ✓ | ||||
| Regeneration of peripheral neurons | ✓ | ✓ | ✓ |