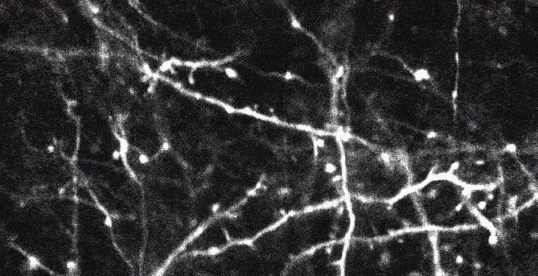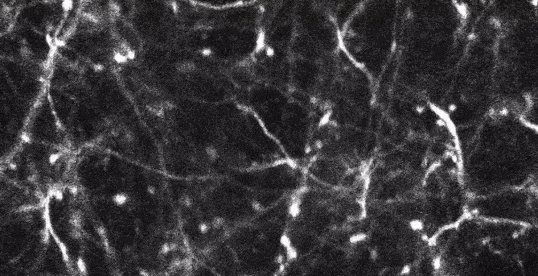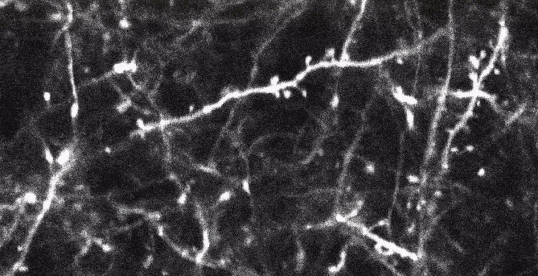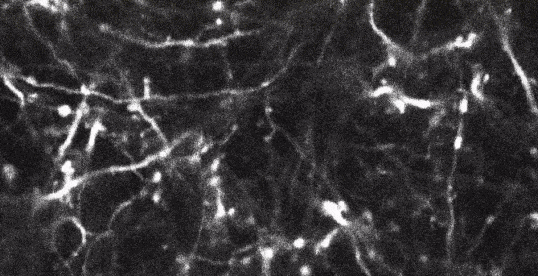
Two-photon imaging of fluorescent neurons in the brain of a living mouse. Data obtained at Neurotar.
We use two-photon microscopy to measure dendritic spine turnover in the most physiologically relevant model system: the brains of awake, behaving mice. Typical readouts are dendritic spine density, formation rate, elimination rate, mobile spine fraction, and net change. Our assay is uniquely suited to assess drug efficacy or mode of action longitudinally or to characterize mouse disease models. We can combine this assay with video recordings (e.g. of pupil dilation) or simple behavioral readouts.
Dendritic spine turnover drives memory and learning through neural rewiring, and is associated with a variety of neurological disorders
Dendritic spines are small protrusions on dendrites that form synapses with neighboring axons. They display unique plasticity, with new dendritic spines constantly being formed all around the brain while existing spines are removed. Spine plasticity is essential to rewire neural circuits, which is the primary mechanism for learning and memory. Importantly, defects in dendritic spine turnover are associated with several neurological disorders, such as depression, Alzheimer’s disease, Parkinson’s disease, and intellectual disability. An interesting new trend is to treat neurological disorders with psychedelic drugs, such as Psilocybin, LSD, DMT, and MDMA. Such drugs can for example promote structural and functional neural plasticity, produce antidepressant-like effects and spur the growth of neural connections lost in depression.
Why study dendritic spine dynamics using two-photon microscopy?
Dendritic spine dynamics can be studied using a variety of in vitro and ex vivo assays. Common assays include imaging of cultured neuronal cells or brain slices using confocal microscopy. While having generated important insights, these simple methods do not capture the complexity of a living organism. Therefore, the relevance of data obtained in these in vitro preparations is limited.
Two-photon microscopy enables high-resolution imaging of living tissue at great imaging depth with low phototoxicity (for more information, please visit our two-photon page). For that reason, it is the only technology to image dendritic spines and quantify their dynamics (turnover) in the brains of living mice. Since two-photon microscopy is non-invasive, the same part of the brain in the same animal can be re-examined for up to several months. In addition, control data can be obtained from the same animal, which reduces variability and allows smaller group sizes. Therefore, performing dendritic spine turnover experiments using two-photon microscopy Reduces the number of animals and Refines data quality. This complies with the 3R principle for the use of experimental animals.
Stack of two-photon images of Layer V neurons of the cortex in Thy1-YFP mouse
How can Neurotar facilitate your evaluation of dendritic spine turnover?
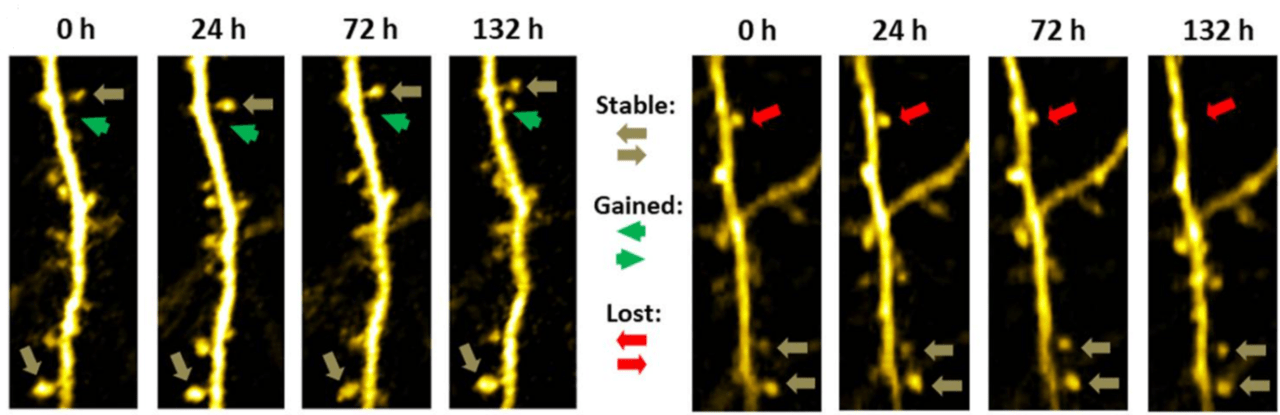
Neurotar is the world’s leading commercial service provider of in vivo two-photon brain imaging, the only technique to visualize dendritic spines and quantify their dynamics (turnover) in the brains of living mice. Our expert team has completed multiple dendritic spine turnover studies for a variety of companies from all over the globe. Typical readouts for these studies are dendritic spine density, formation rate, elimination rate, mobile spine fraction, and net change.
Importantly, as ketamine and other general anesthetics strongly affect spine turnover, spine imaging studies at Neurotar can be performed in awake, behaving mice. Such studies are enabled by our proprietary Mobile HomeCage, which combines stable head fixation with a flat-floored air-lifted cage to allow precise imaging in the brain of mice freely moving in 2D.
Typical experimental setup
- 2-5 groups, e.g., 1 placebo, 1-2 test compounds, and optionally 1 positive control and/or 1 negative control
- 3-5 imaging sessions: baseline at 0 h, 2-4 imaging sessions over several days, weeks, or months
- 1-3 imaging areas per animal
- We quantify turnover of at least 150 individual dendritic spines per animal
Mouse Models
We can accommodate most transgenic mouse models in our studies. For example, we have used the Thy1-YFP mouse line that expresses YFP in Layer V neurons of the cortex. We can also utilize mouse models for various neurological diseases, such as Alzheimer’s Disease (for example P301S and APP/PS1) and Parkinson’s disease (for example LRRK2). In such disease models, viral vectors can be used to visualize neurons. Neurotar can negotiate the commercial license required for such studies with the licensor on a client’s behalf. Alternatively, clients can extend their license to Neurotar for the duration of the study.
Options and extensions
- Drug administration through a variety of routes, for example oral (gavage), through i.p., s.c., i.v. or i.t. (intrathecal) injection, or through injection into the Cisterna Magna.
- We can combine experiments with pharmacokinetics.
- In addition to dendritic spine turnover measurements, we can perform simple behavioral readouts, for example, von Frey nociception assays and hot plate tests.
- We can study dendritic spine turnover under several brain injury conditions, such as severe global ischemia and focal stroke.
- Additional readouts can be added to the study, for example, mitochondrial fragmentation.
- After the experiment has finished, we can harvest and preserve tissues for further analyses.
Other two-photon brain imaging services offered by Neurotar
| Neurodeg. Disease (e.g. AD, PD) | Stroke and TBI | Neuropathic Pain and Migraine | Neuropsychiatry (e.g. Schizophrenia) | Epilepsy | |
|---|---|---|---|---|---|
| Blood-brain barrier integrity | ✓ | ✓ | ✓ | ||
| Trans-BBB pharmacokinetics | ✓ | ✓ | ✓ | ||
| Dendritic spine turnover | ✓ | ✓ | ✓ | ✓ | ✓ |
| Microglial dynamics or response to injury | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium signaling | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abeta Plaque or Tau Tangle dynamics | ✓ | ✓ | ✓ | ||
| Mitochondrial fragmentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ischemic Stroke model | ✓ | ||||
| Regeneration of peripheral neurons | ✓ | ✓ | ✓ |
References
- Pryazhnikov E, Mugantseva E, Casarotto P, Kolikova J, Fred SM, Toptunov D, Afzalov R, Hotulainen P, Voikar V, Terry-Lorenzo R, Engel S, Kirov S, Castren E, Khiroug L. (2018) Longitudinal two-photon imaging in somatosensory cortex of behaving mice reveals dendritic spine formation enhancement by subchronic administration of low-dose ketamine. Sci Rep. 24;8(1):6464. DOI: 10.1038/s41598-018-24933-8.
- Kislin M, Sword J, Fomitcheva IV, Croom D, Pryazhnikov E, Lihavainen E, Toptunov D, Rauvala H, Ribeiro AS, Khiroug L, Kirov SA. (2017) Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J Neurosci 11;37(2):333-348. DOI: 10.1523/JNEUROSCI.1510-16.2016.
- Kasai H, Ziv NE, Okazaki , Yagishita S, Toyoizumi T. (2021) Spine dynamics in the brain, mental disorders and artificial neural networks. Nature Reviews Neuroscience 22(7):407-422. DOI: 10.1038/s41583-021-00467-3.
- Runge K, Cardoso C, de Chevigny A. (2020) Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci., 12:36. DOI: 10.3389/fnsyn.2020.00036.
- Ma S, Zuo Y. (2021) Synaptic modifications in learning and memory – A dendritic spine story. Seminars in Cell & Developmental Biology May 18;S1084-9521(21)00124-5. DOI: 10.1016/j.semcdb.2021.05.015.
- Cameron LP, Tombari RJ, Lu J, …, Olson DE. (2021) A Non-Hallucinogenic Psychedelic Analog with Therapeutic Potential. Nature Jan; 589(7842): 474–479. DOI: 10.1038/s41586-020-3008-z
- Gipson CD, Olive MF. (2017) Structural and functional plasticity of dendritic spines – root or result of behavior? Genes Brain Behav. 2017 Jan;16(1):101-117. DOI: 10.1111/gbb.12324.
- Forrest MP, Parnell E, Penzes P. (2018) Dendritic structural plasticity and neuropsychiatric disease; Nat Rev Neurosci. Mar 16; 19(4): 215–234. DOI: 10.1038/nrn.2018.16.
- Nishiyama J. (2019) Plasticity of dendritic spines: Molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin. Neurosci. 73:541–550. DOI: 10.1111/pcn.12899.
- Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, Kwan AC. (2021) Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo; Neuron Aug 18;109(16):2535-2544.e4. DOI: 10.1016/j.neuron.2021.06.008.
- Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Zarandi SS, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister K, Ori-McKenney KM, Gray JA, Olson DE. (2018) Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. Jun 12; 23(11): 3170–3182. DOI: 10.1016/j.celrep.2018.05.022.
- Lukasiewicz K, Baker JJ, Zuo Y, Lu J. (2021) Serotonergic Psychedelics in Neural Plasticity. Front. Mol. Neurosci., Oct 12;14:748359. DOI: 10.3389/fnmol.2021.748359.
- Vann Jones SA, O’Kelly A. (2020) Psychedelics as a Treatment for Alzheimer’s Disease Dementia. Front. Synaptic Neurosci., Aug 21;12:34. DOI: 10.3389/fnsyn.2020.00034.
- Khan SM, Carter GT, Aggarwal SK, Holland J. (2021) Psychedelics for Brain Injury: A Mini-Review. Front. Neurol., Jul 29;12:685085. DOI: 10.3389/fneur.2021.685085.
- Subramanian J, Savage JC, Tremblay ME. (2020) Synaptic Loss in Alzheimer’s Disease: Mechanistic Insights Provided by Two-Photon in vivo Imaging of Transgenic Mouse Models. Front Cell Neurosci. Dec 17;14:592607. DOI: 10.3389/fncel.2020.592607.
- Phoumthipphavong V, Barthas F, Hassett S, Kwan AC (2016) Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. ENeuro Apr 4;3(2). 10.1523/ENEURO.0133-15.2016.