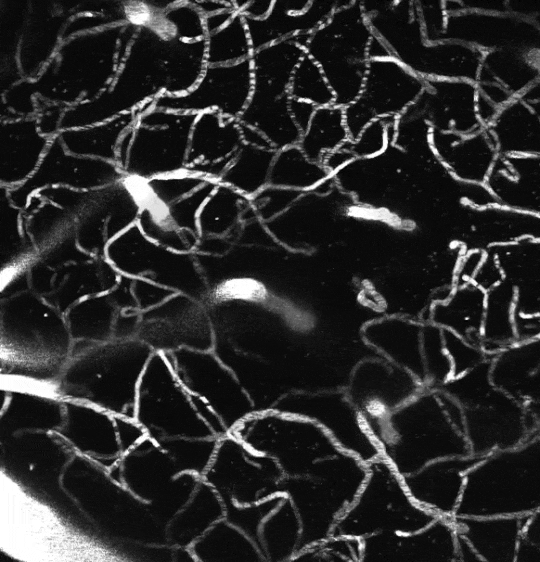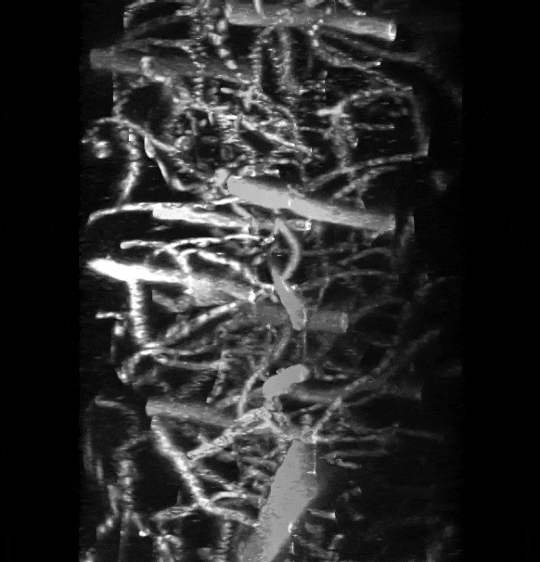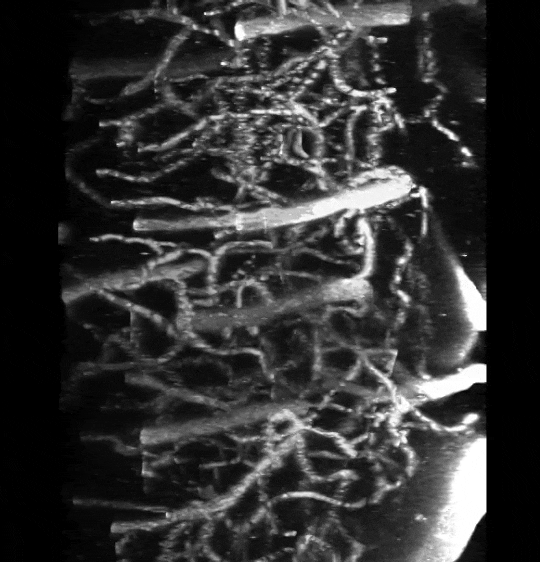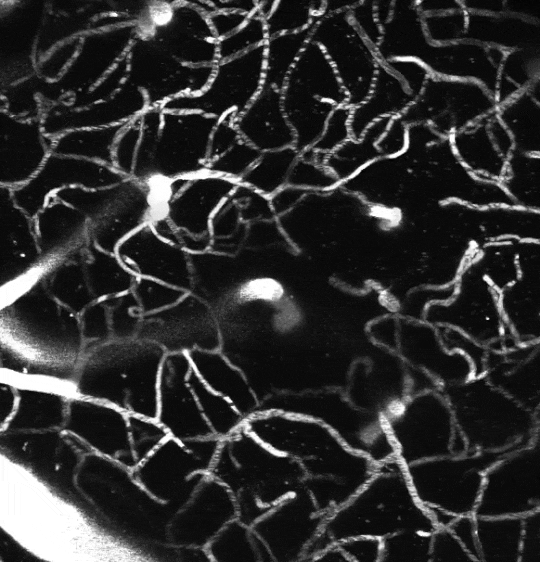
Two-photon image of the vasculature in the somatosensory cortex of a living mouse. Image obtained at Neurotar.
Trans-BBB Pharmacokinetics
Neurotar offers a unique assay that measures your lead compound’s ability to cross the blood-brain barrier (trans-BBB pharmacokinetics). We do this using two-photon microscopy in the most physiologically relevant model: the brain of a living mouse. Our scientists measure trans-BBB pharmacokinetics longitudinally and can combine these measurements with efficacy assessments, localization studies, and various other readouts.
The blood-brain barrier (BBB) poses a challenge to drug discovery
The blood-brain barrier poses a challenge to drug development as it prevents the majority of drugs, especially biologics, from reaching the brain. This barrier may be beneficial for drugs that do not target CNS disorders, as it prevents the brain from potential adverse effects. However, it complicates drug development for CNS disorders, brain cancer, and brain metastases. To overcome this challenge, several approaches have been developed to bypass the BBB, many of which use nanoparticles or ultrasound. Therefore, regardless of the indication, it is important to quantitatively assess the capability of new drug candidates to cross the BBB. This is done using trans-BBB pharmacokinetic (PK) assays.
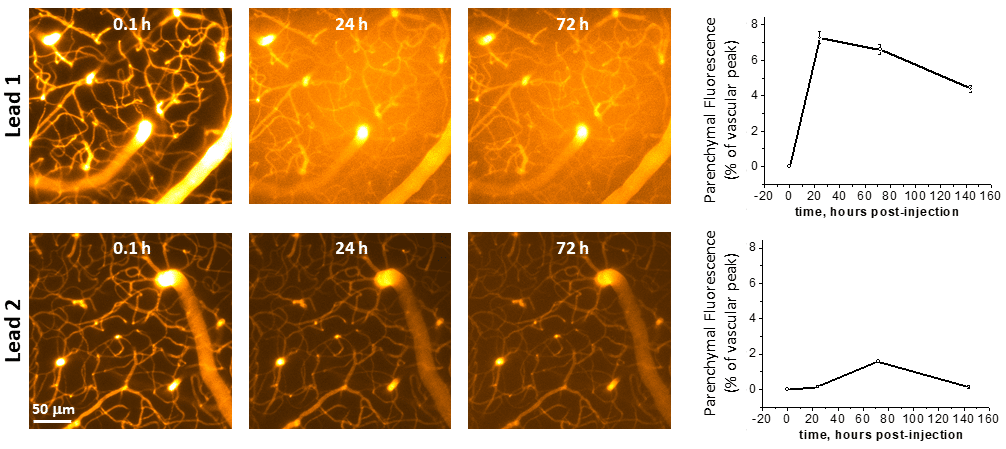
Example results from a trans-BBB PK study performed at Neurotar. The vasculature of the somatosensory cortex was imaged using two-photon microscopy at the indicated time points after injection of fluorescently labeled lead compounds. Quantification of parenchymal and vascular fluorescence demonstrated that Lead 1 very effectively crosses the BBB.
Why study trans-BBB pharmacokinetics using two-photon microscopy?
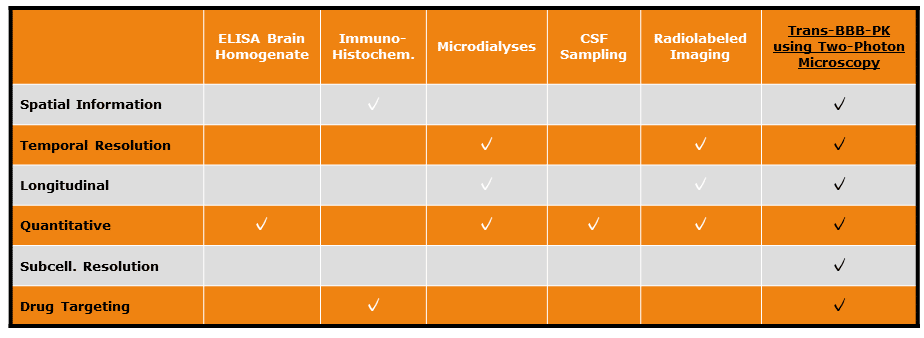
Cell culture-based in vitro assays that mimic the BBB are too simple to faithfully model the complex, dynamic environment of the living brain. Therefore, the best way to study whether a compound can cross the BBB is in the brain of a living organism. Multiple assays exist to assess trans-BBB PK properties in mice. The majority of these methods, however, have severe limitations (see table below).
Two-photon microscopy is an ideal method to perform trans-BBB PK studies. Images can be obtained in the brains of living mice at high resolution (up to organelle level) and such images can be reliably quantified. In addition, due to low phototoxicity, longitudinal follow-up of the same brain region in the same mouse is possible for up to several months. To read more about the benefits of two-photon microscopy for in vivo brain imaging and how it complies with the 3R principle for animal research, please visit our dedicated web page.
How can Neurotar help you?
Neurotar is the world’s leading commercial service provider of in vivo two-photon brain imaging in mice. We have developed and validated a unique assay to measure the ability of lead compounds, most often antibodies or other biologics, to cross the BBB. The fluorescently-labeled compound is injected intravenously after which the same brain region is repeatedly imaged. From the resulting images, redistribution through the BBB into the cortical parenchyma is ratiometrically quantified. We have performed several such studies for a variety of commercial customers.
In addition to the ability to cross the BBB, we can also assess where the lead compound accumulates after crossing the BBB or whether the compound is biologically active. For example, in an Alzheimer’s disease model, we can determine whether an antibody targeting Abeta peptides accumulates in amyloid plaques after crossing the BBB (see example data below). In addition, we can perform efficacy measurements by determining whether the lead compound reduces amyloid plaque burden (please check our Abeta plaque dynamics page for details).
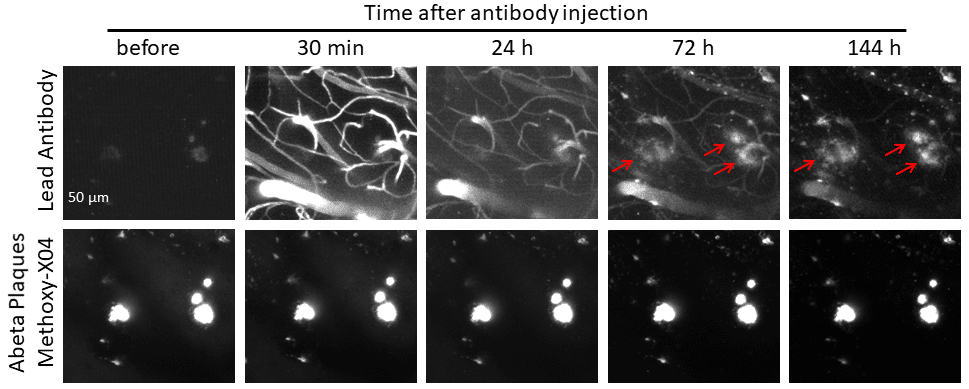
Example of a combined BBB PK and Abeta plaque targeting study performed at Neurotar. The somatosensory cortex of a P301S mouse (model for Alzheimer’s) was imaged using two-photon microscopy at the indicated time points after injection of a fluorescently labeled antibody. The data at 24h demonstrate that the lead antibody crosses the BBB, while images obtained at 72h and 144h show targeting of the antibody to Abeta plaques.
Typical experimental setup for a trans-BBB pharmacogenetics study
- 1-2 groups, e.g., the test compound and, potentially, a control compound
- 4 imaging sessions (for example, 0-1 h, 24 h, 72 h, and 144 h), but the same region can be followed for months
- 1-3 imaging areas per animal
- 6-14 regions of interest (ROIs) measured per imaging area, in blood vessels and parenchyma
Mouse Models
We perform most studies in WT mice. However, we can accommodate most transgenic mouse models. For example, we can use Alzheimer’s mouse models, like P301S and APP/PS1, to simultaneously study the trans-BBB PK of a lead compound and its ability to target Tau Tangles or Abeta plaques. Neurotar can negotiate the commercial license with the licensor on a client’s behalf. Alternatively, clients can extend their license to Neurotar for the duration of the study.
Options and extensions
- We can combine the BBB PK measurements with other readouts such as quantification of Abeta plaques, Tau tangles, dendritic spine turnover, or calcium signaling.
- We can perform behavioral tests, such as a hot plate experiment or von Frey nociception assay.
- After the experiment has finished, we can harvest and preserve tissues for further analysis.
Other two-photon brain imaging services offered by Neurotar
| Neurodeg. Disease (e.g. AD, PD) | Stroke and TBI | Neuropathic Pain and Migraine | Neuropsychiatry (e.g. Schizophrenia) | Epilepsy | |
|---|---|---|---|---|---|
| Blood-brain barrier integrity | ✓ | ✓ | ✓ | ||
| Trans-BBB pharmacokinetics | ✓ | ✓ | ✓ | ||
| Dendritic spine turnover | ✓ | ✓ | ✓ | ✓ | ✓ |
| Microglial dynamics or response to injury | ✓ | ✓ | ✓ | ✓ | ✓ |
| Calcium signaling | ✓ | ✓ | ✓ | ✓ | ✓ |
| Abeta Plaque or Tau Tangle dynamics | ✓ | ✓ | ✓ | ||
| Mitochondrial fragmentation | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ischemic Stroke model | ✓ | ||||
| Regeneration of peripheral neurons | ✓ | ✓ | ✓ |
References
- Hoehlig K, Johnson K, Pryazhnikov E, Maasch C, Clemens-Smith A, Purschke W, Vauléon S, Buchner K, Jarosch F, Khiroug L, Vater A, Klussmann S. (2015) A novel CGRP-neutralizing Spiegelmer attenuates neurogenic plasma protein extravasation. Br J Pharmacol. DOI: 10.1111/bph.13110.
- Kadry H, Noorani B, Cucullo L. (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. Nov 18;17(1):69. DOI: 10.1186/s12987-020-00230-3.
- Pardridge WM. (2020) Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front Aging Neurosci. Jan 10;11:373. DOI: 10.3389/fnagi.2019.00373.
- Bhowmik A, Khan R, and Ghosh MK. (2015) Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. Biomed Res Int. 2015: 320941. Mar 19. DOI: 10.1155/2015/320941.
- Arvanitis CD, Ferraro GB, Jain RK. (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nature Reviews Cancer. 20:26–41. DOI: https://doi.org/10.1038/s41568-019-0205-x.
- Pandit R, Chen L, Götz J. (2020) The blood-brain barrier: Physiology and strategies for drug delivery. Adv Drug Deliv Rev. 165-166:1-14. DOI: 10.1016/j.addr.2019.11.009.
- Ferraris C, Cavalli R, Panciani PP, Battaglia L. (2020) Overcoming the Blood-Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. Int J Nanomedicine. Apr 30;15:2999-3022. DOI: 10.2147/IJN.S231479.
- Parvez S, Kaushik M, Ali M, Alam MM, Ali J, Tabassum H, Kaushik P. (2022) Dodging blood brain barrier with “nano” warriors: Novel strategy against ischemic stroke. Theranostics. Jan 1;12(2):689-719. DOI: 10.7150/thno.64806.
- Abrahao A, Meng Y, Llinas M, Huang Y, …, Zinman L, (2019) First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. Sep 26;10(1):4373. DOI: 10.1038/s41467-019-12426-9.
- Williams-Medina A, Deblock M, Janigro D. (2021) In vitro Models of the Blood–Brain Barrier: Tools in Translational Medicine. Front Med Technol. 2020; 2: 623950. Feb 15. DOI: 10.3389/fmedt.2020.623950.
- Kucharz K, Kutuzov N, Zhukov O, Mathiesen Janiurek M, Lauritzen M. (2022) Shedding Light on the Blood–Brain Barrier Transport with Two-Photon Microscopy In Vivo. Pharmaceutical Research. https://doi.org/10.1007/s11095-022-03266-2.